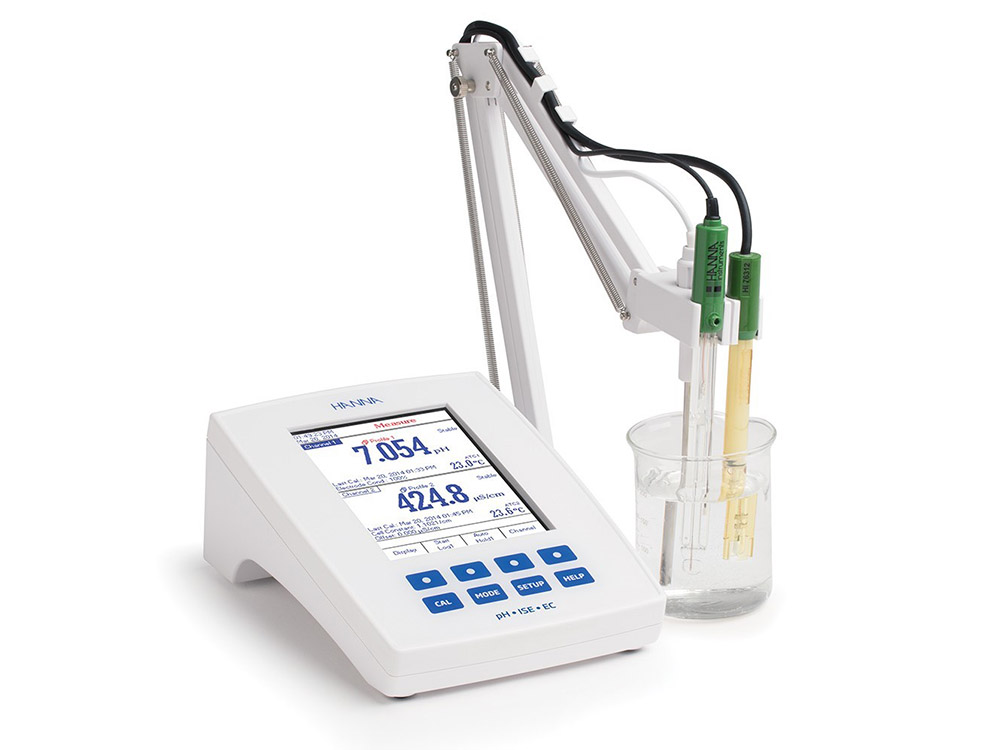
pH
Background:
A pH (potential of Hydrogen) measurement reveals if a solution is acidic or alkaline, or neutral. The pH scale is logarithmic and runs from 0.0 (acidic) to 14.0 (alkaline or basic) with 7.0 being neutral.
Listeria spp. in environmental samples collected from production facilities, especially if ready-to-eat products are being produced.
Method pH water
APHA SM 4500-H+B ISO Accredited
Method pH food
AOAC 981.12, USDA/FSIS MLG Ch 2 ISO Accredited
Method pH dairy
APHA SMEDP 15.022 ISO Accredited
apparatus
pH meter with a resolution of 0.01 pH units with combination glass electrode
turn around time
Same day if submitted before 1 pm
sample required
Liquid Samples: 100 mL
Solid Samples: 10 to 100 g
Semi-Solid Samples: about 4 oz
type of test
Potentiometric
reportable units
pH
measurement range
0.0 to 14.0
Related Resources
Analysis Description:
Samples are prepped according to various techniques which are appropriate to the sample submitted.
1. Sensing bulb
2. Glass (or measuring, sensor) calomel electrode
3. Internal Solution (saturated, neutral KCL)
4. N/A
5. Reference calomel electrode
6. Reference internal solution (saturated, neutral KCL)
7. Ceramic junction
8. Body of Electrode
The glass electrode is the pH-sensing electrode (#2). During measurements, an electrical potential is produced across the thin “glass membrane” tip of the glass electrode, which is a bulb of special glass that is electrically conductive (#1). The rest of the glass body of the electrode is non-conductive. The potential is proportional to the H+ concentration, and the pH meter converts the potential differences directly into pH units.
The reference electrode (#5) serves as a stable potential standard for comparison with the glass electrode. It also completes the electrical measuring circuit by using saturated KCl (#6) as a liquid junction to do so. To complete the circuit, a very small amount of this electrolyte solution must pass through a very small hole (ceramic junction #7) at the bottom of the electrode continuously while pH measurements are being made. Proper flow of KCl requires that an air hole near the top of the electrode be opened up prior to making measurements.

